
Several medical devices including blood bags, surgical stitches, contact lenses, safety glasses and some respiratory equipment also have the application of chlorine chemistry in their manufacturing process.Ĭhlorine is used in the process of manufacturing various devices used in the extraction of renewable energy resources. In the manufacture of many medicines including those to control cholesterol, fix allergy symptoms and relieve arthritis pain use of chlorine is crucial. The chemistry of chlorine is quite important in pharmaceutical industries. Germs found on household surfaces causing stomach bugs (norovirus), seasonal flu and other diseases can be easily killed with diluted bleach solutions. coli, salmonella and hosts of some other foodborne germs are disinfected easily with chlorine-based disinfectants.Ĭhlorine is widely used in manufacturing bleaches which is further very helpful in cleaning, disinfecting and brightening clothes, kitchen and washroom surfaces.
CHLORINES ATOMIC RADIUS FREE
It is also useful in keeping the kitchen surfaces germ free and safe to prepare food on them.
CHLORINES ATOMIC RADIUS SKIN
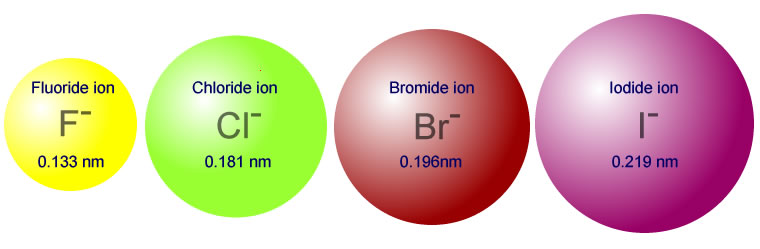
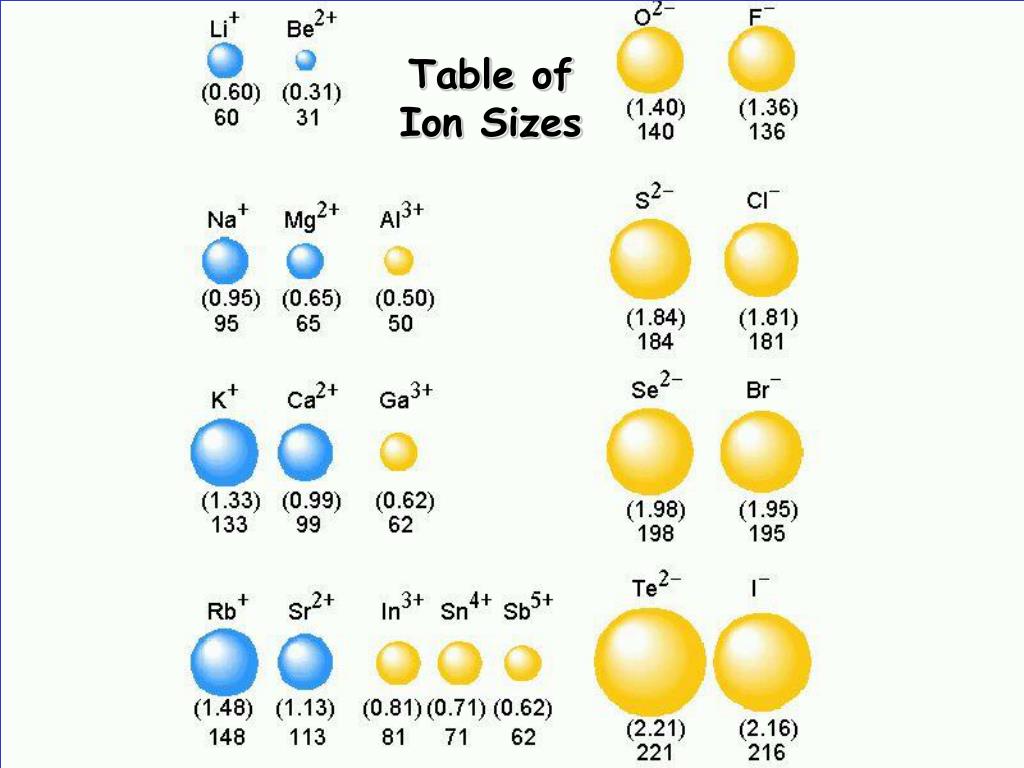
This way, it prevents diseases like diarrhoea, rashes on the swimmer’s ear or skin etc.Ĭhlorine plays an important role in the abundant production of crops by protecting them against pests. Chlorine-based disinfectants for pools and spas are really helpful in killing waterborne pathogens and keeping the water safe from causing illness. Before chlorine-based disinfectants became popular, waterborne diseases like typhoid, dysentery, cholera and hepatitis A took thousands of lives every year. The chemistry of chlorine has helped to a large extent in keeping the drinking water and water in pools safe. Hypochlorites (salts of hypochlorous acid), chlorites and chlorates also contain chlorine in the oxidised state Mixtures of chlorine and hydrogen in some specific proportions become explosiveĬl 2 O, ClO 2, O 2 O 6, Cl 2 O 7 and Cl 2 O 8 are some oxides of chlorine. The reaction of chlorine with alkali metals occurs with combustion in the presence of tiny amounts of moisture Polyvinyl chloride (PVC), hydrochloric acid and Sodium chloride (common salt)ĭry chlorine is reactive towards most of the metals but only upon heating Properties of Chlorine Physical Properties Some of the physical and Chemical properties of Chlorine are listed below. As the outermost shell has one electron less from attaining the nearest noble gas configuration, its tendency is to form an anion ‘Cl - ’. The outermost shell contains 7 electrons in total. The electronic configuration of chlorine is 1s 2 2s 2 2p 6 3s 2 3p 5 (or \\] 3s 2 3p 5 ).Īs depicted in the picture, the first and second electron shells of chlorine atoms are completely filled up with 2 and 8 electrons respectively. Chlorine formula (chemical formula of chlorine) is Cl 2. Chlorine is present in gaseous form at room temperature with a yellowish-green appearance. Chlorine belongs to group 17 (or VII-A) elements of the periodic table and the symbol of chlorine is ‘Cl’.īeing the second lightest halogen, it is positioned between fluorine and bromine in the periodic table and its properties are mostly intermediate between them.
CHLORINES ATOMIC RADIUS FULL
Chlorine is also denoted with the symbol Cl (Cl full form is chlorine). Chlorine got its name in 1810 by Humphry Davy, who claimed that it was in fact an element. Initially, he mistakenly thought it contained oxygen. Chlorine is a chemical element that was discovered in 1774 by Carl Wilhelm Scheele.


 0 kommentar(er)
0 kommentar(er)
